Let’s Make the Most of Our Time at IRT 2025!
Connect With Us!
Linking you to the latest in research pharmacy and IDS best practices
- Event: IRT 2025
- Date: October 14-15, 2025
- Location: The Westin Copley Place, Boston
- Speaker: Cherish Lallone
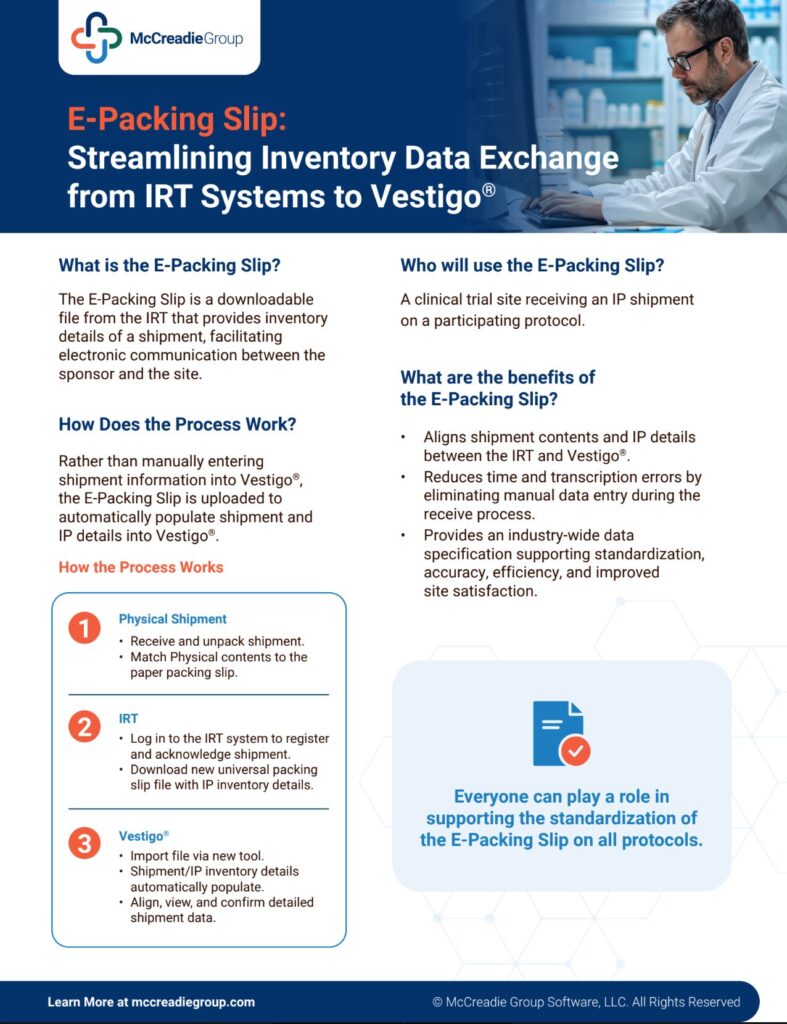
Simplify Your Clinical Trial Shipment Management
Streamline your investigational drug shipments with our free E-Packing Slip File Standard. This open-source data standard automates inventory data integration between RTSM systems and research sites’ electronic inventory platforms, reducing manual entry, minimizing errors, and improving efficiency by up to 50%.
Learn More about this simple, high-value solution and start automating your inventory management today.

Meet The Team

Cherish Lallone, MS
Vice President, Business Solutions at McCreadie Group Speaker
Cherish is a seasoned technology leader with a decade of experience at McCreadie Group, specializing in Vestigo and PharmAcademic product development. Her background in manufacturing, automotive, and robotics equips her with a deep understanding of industry challenges. At McCreadie Group, Cherish drives innovative, tech-based solutions that boost efficiency in the pharmacy sector, focusing on systems design, integrations, process optimization, and data analytics to deliver value to clients.

Sara Brueck Nichols, MHA
President
Sara joined McCreadie Group in 2024 from Optum Rx, a UnitedHealth Group company. She is responsible for driving growth, product, and service strategy, and comes with nearly 25 years of experience in strategic planning, operational optimization, and data-driven decision making. For the last 8.5 years, she has focused on the intersection of marketing, operations, and technology in the pharmacy services space: including data and analytics, scalability, efficiency, transformation, and privacy. Sara holds a Master of Healthcare Administration from the University of Utah’s Eccles School of Business.

Rachael Aletti, PharmD
Solutions Engineer
Rachael joined McCreadie Group in 2019 and is now a solutions engineer, drawing on her experience in account management, business development, and research pharmacy to support clients and demonstrate Vestigo’s value. A licensed pharmacist with over 13 years of experience, she began in inpatient care before moving into investigational drug services in 2013, with training and practice at Montefiore Medical Center and NYP–Weill Cornell.
Learn More About Vestigo
Vestigo automates Investigative Drug Services (IDS) operations, boosting safety, efficiency, and compliance in clinical trials.
Subscribe to McCreadie Group Connect
Linking you to the latest in research pharmacy and IDS best practices